Abstract:
There a variety of testing methods for COVID-19 testing being advocated by different governments
and healthcare institutions. This article provides an overview of the various techniques available
and their respective advantages and applications.
Introduction:
COVID 19 seems to be the most popular and visible word for the year 2020. An epidemic of global
scale which has brought almost all the countries and mankind to a standstill. The enigma about
the novel corona virus strain, its rapid spread across continents coupled with a wide scale analysis
of all aspects on pandemic has led to a plethora of questions and confusions about state of
affairs, possible trajectories/scenarios, response options and potential solutions. The jury is still
out and will likely to remain busy for months to come before they can conclude what went right
and wrong. Meanwhile, governments, policy makers as well as private organizations and
individuals continue to make decisions with limited knowledge and adjust continuously along
with new found information and evidence.
In such times, testing for Corona virus infection has emerged as a very critical and controversial
topic as testing forms the basis of confirming the infection and learning about its characteristics
and spread. Since testing is very crucial, it was evoked a lot of interest, investments, interventions
from policy makers and resultant dialogues and debates.
Testing Options
The testing tools and techniques have been continuously evolving along the way. The primary
testing tools include:
- NAAT or Nucleic Acid Amplification Tests are considered the gold standard for viral detections as these deals directly with the DNA/RNA of pathogens present in the patient sample. The quantification of the viral load or copy numbers further help in monitoring the progression of the disease and response to therapy. are considered the gold standard for viral detections as these deals directly with the DNA/RNA of pathogens present in the patient sample. The quantification of the viral load or copy numbers further help in monitoring the progression of the disease and response to therapy. NAAT tests are performed using a variety of approaches such as Real Time – PCR (RT-PCR), Isothermal Amplification (LAMP), Micro NMR (µNMR), Next Generation Sequencing (NGS) etc. B. Serology or Antigen/Antibody testing is a technique to rapidly evaluate the infection by testing for proteins produced by the body in response to the infection. These antibodies are typically made within 1 to 3 weeks after infection. The primers for NAAT testing can be designed to cover one or more specific loci of the gene and thereby can used for very targeted detection and differentiation of COVID-19 or SAR2-CoV-2 with other types of Corona viruses. The NAAT test promise very high sensitivity for detection even in instances of very low copy numbers (typically > 50 copies). The main challenges with NAAT include the higher cost of setup and per sample run, technical complexities of performing the tests and turnaround time (TAT). Despites its higher sensitivity, there have been issues of false positive or negatives due to sample specific issues, pre-analytical errors or cross contaminations. Even then, NAAT remains the most potent tool in the fight against COVID-19.
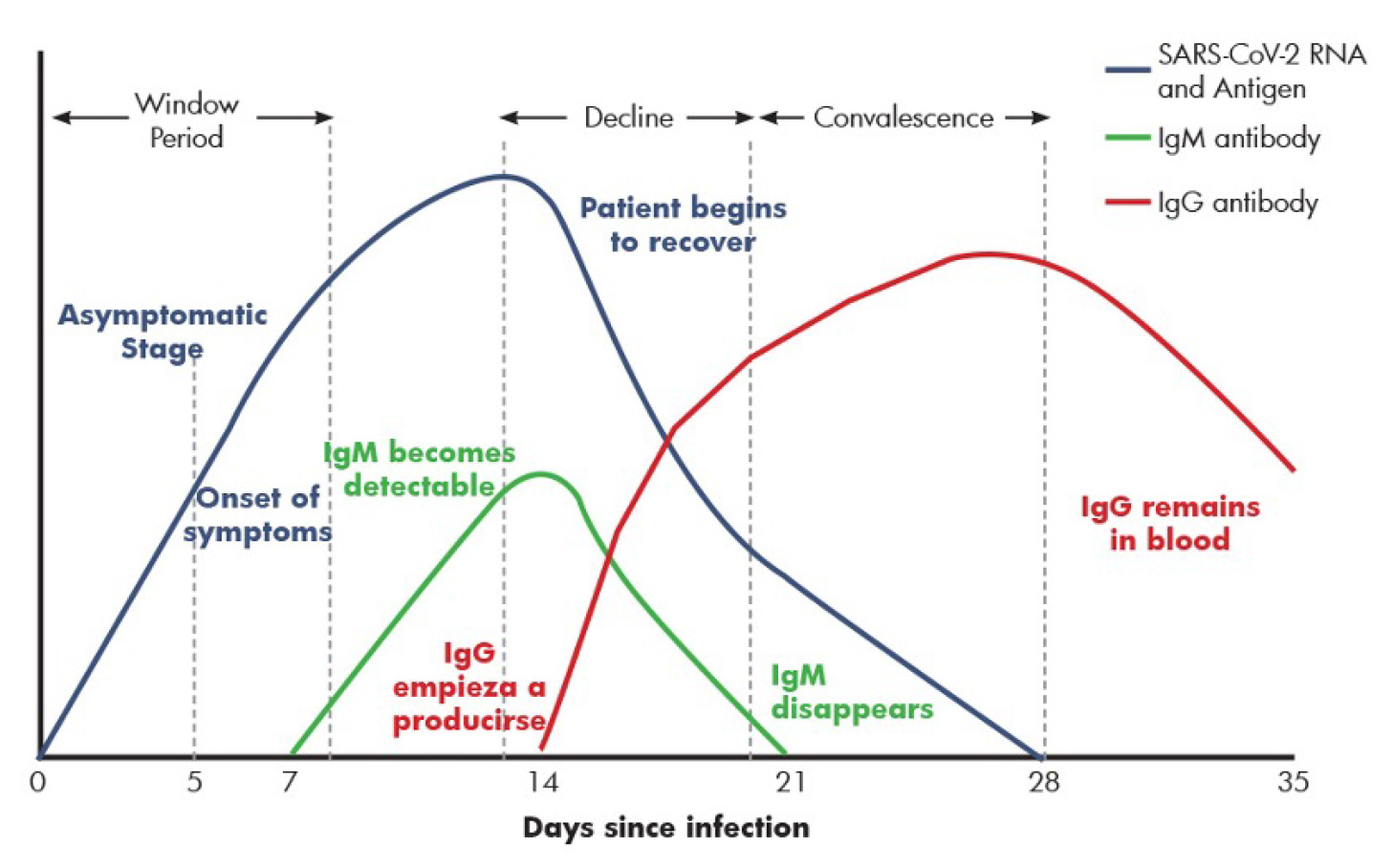
- Serology or Antigen/Antibody Testing is a technique to rapidly evaluate the infection by testing for proteins produced by the body in response to the infection. These antibodies are typically made within 1 to 3 weeks after infection. Antibodies are Y-shaped proteins that can recognize Antigens or foreign particles whereby Antibodies target and eliminate them. A specific antibody recognizes and binds to its corresponding antigen. Antigens, also known as immunoglobulins can be a protein, polysaccharides or lipids. Similarly there are different classes of antibodies (IgG, IgM, IgA) each having a unique role in immunity. It takes 1-2 weeks post symptom onset for patients to seroconvert to SARS-CoV-2.
- Other Diagnostic Tools: While NAAT and Serological testing are the appropriate testing mechanisms for presence or absence of virus or the antibodies, there are several criteria defined for case selection for testing. Additional challenges of affordability and accessibility means that the entire population cannot be subjected to COVID testing in a short time. Hence the use of various other diagnostic tools for identification and monitoring of suspected cases. These include blood gas analyzers, CT and X-rays amongst others.
- Testing for Co-Morbidities: Analysis of corona related fatality rates across geographies have highlighted the role of co-morbidities in adverse outcomes. Fatality rates are typically higher in older populations, immune compromised patients (e.g. cancer or transplant cases) or those with other disorders. Patients with hypertension, diabetes or cardiovascular disorders were found to be developing more severe symptoms leading to higher hospitalization and fatality rates. Hence the routine surveillance of such patients is useful in early identification of high risk cases and better management of infected patients.
polysaccharides or lipids. Similarly there are different classes of antibodies (IgG, IgM, IgA)
each having a unique role in immunity. It takes 1-2 weeks post symptom onset for patients
to seroconvert to SARS-CoV-2.
Source: https://www.hcmarbella.com/en/techniques-for-an-accurate-and-early-diagnosis-of-covid-19/
By detecting the presence of antibodies to SARS-CoV-2 in the patient, one can judge the
immune response irrespective of symptomatic or asymptomatic state of the patient. And the
nature of immune response can help distinguish a recent vs. an older infection. Hence, serology
tests play an important role in management and surveillance of the disease and have several
applications in patient care as well as epidemiology.
By detecting the presence of antibodies to SARS-CoV-2 in the patient, one can judge the immune
response irrespective of symptomatic or asymptomatic state of the patient. And the nature of
immune response can help distinguish a recent vs. an older infection. Hence, serology tests play
an important role in management and surveillance of the disease and have several applications
in patient care as well as epidemiology.
Serology assay are relatively less complex, easier to perform, cheaper and faster as compared
to NAAT. Hence these can be widely used. However the main challenge with serology testing is
lower sensitivity & specificity leading to higher false negative and false positive reports.
Hence Serology is not recommended for primary detection of the infection. Although it has an
important role in post infection monitoring and surveillance
Radiology techniques are useful in early identification and monitoring the disease progression, given that
COVID is a respiratory disorder and many patients end up with lung or related discomforts. There have
been some claims related with improved speed and sensitivity of radio diagnosis techniques in diagnosis
of corona cases. Yet these techniques are most useful in hospital settings while managing patients under
treatment and those with acute infections and respiratory complications.
From testing perspectives, although testing for co-morbidities has been part of routine healthcare, it has
more significance in the present COVID times given the impact on patient outcomes and generally
stretched healthcare infrastructure.
Testing Challenges
From testing perspectives, although testing for co-morbidities has been part of routine healthcare, it has
more significance in the present COVID times given the impact on patient outcomes and generally
stretched healthcare infrastructure.
Accuracy: As described in the previous section, there are known differences in the sensitivity/specificity
of various techniques. And yet each approach has its advantages, applications and use cases. Most
countries have adopted NAAT as the primary testing option supported by Serology based surveillance.
The test sensitivity varies as per the duration of illness as the actual viral load varies over time and
antibodies are generated only after certain period from the date of infection. Further the actual mutation
and the assay design can also have a bearing on the sensitivity in practice. Sensitivity is also affected by
pre-analytical factors like type, site and quality of specimen collected. Hence there have been reports
of false positive/negative cases even in NAAT testing though it is a very sensitive assay.
Capacity: The key challenge for most countries has been the testing capacity. The rapid advent and
spread of the disease across geographies and all strata of population has led to an
exponetiatial rise in the testing needs. Most countries adopted preventive measures such a social
distancing and lockdowns to ‘flatten the curve’ of the spread of the disease with varying success. The
primary aim of the preventive measures was the match somewhat the rise in peak with the availability of
hospital beds/ventilators/ICUs etc. However, testing has remained the primary mechanism for identification
or ruling out of the infection and the subsequent classification of a recovered patient.
Developing such a vast testing infrastructure is a challenge. Especially for RT-PCR setups which have
elaborate infrastructure, equipment and manpower requirements. There has been a shortage of RT-PCR
machines and other lab equipments. On top of this, the consumables and testing kits have also been in
short supply. The lockdown leading top roduction and logistics disruptions further exacerbated the problem.
Changing Regulations: Regulators worldwide have had to step-up to managing the sudden influx of new
technologies and providers across all the technologies. Most of the regulators managed to create provisions
for ‘emergency use authorizations and fast track approvals but not without complains and criticisms from
various stakeholders. Validating new technologies, methods and kits is a time consuming process and
doing so in a rushed fashion in bound to have mistakes and loose ends.
Different countries and regulatory bodies have been recommending various criteria for referring of patients
for NAAT testing. This is true especially in India where the government has defined and further refined or
changed the testing criteria several times. The main reason was to prevent a panic or chaos and to avoid
over burdening of the limited testing infrastructure. The guidelines which aimed at limiting the testing raised
a lot more questions than it answered leading to more confusion. For e.g. whether to test asymptomatic
patients or not? Should one test relatives of a patient? Do healthcare workers or police or media qualify
as exposed population and should they be screened? Many of these guidelines are varying from one
state to another.
This has further accentuated the testing availability and caused lot of stress and challenged to analready
stretched healthcare system.
Many technologies are still not available or accessible in India due to lack of regulatory clarity. Example –
there is no clarity if serological testing can be used in general population for screening or in remote locations
where no other testing is available. Till date, it has been allowed only for Zero-survelliance only by the state
governments.
Way ahead
A reduction in public focus, media attention and political interference over time will enable an environment
for more scientific debates and improved regulatory response. The new found focus in healthcare should
attract more private investment leading to greater capacities and innovations. This would be perhaps one
of the few positive impacts of this pandemic in these testing times.
Sources:
https://csb.mgh.harvard.edu/covid
https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html
https://asm.org/Articles/2020/May/COVlD-19-Serology-Testing-Explained
https://www.siemens-healthineers.com/en-in/
https://stm.sciencemag.org/content/scitransmed/12/546/eabc1931. full .pdf
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance

